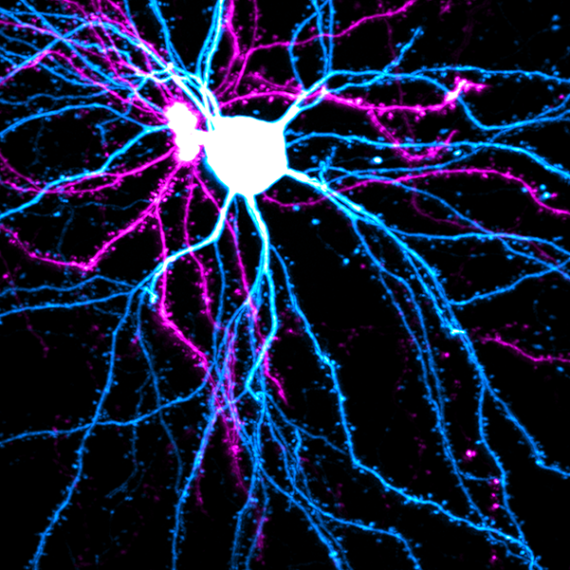
Constructing the striatum
Ann Graybiel has recently uncovered the plan underlying development of the complex architecture of the striatum.
The striatum, the largest nucleus of the basal ganglia in the vertebrate brain, was historically thought to be a homogeneous group of cells. This view was overturned in a classic series of papers from MIT Institute Professor, Ann Graybiel. In previous work, Graybiel, who is also an investigator at MIT’s McGovern Institute, found that the striatum is highly organized, both structurally and functionally and in terms of connectivity. Graybiel has now collaborated with Z. Josh Huang’s lab at Cold Spring Harbor Laboratory to map the developmental lineage of cells that give rise to this complex architecture. The authors found that different functions of the striatum, such as execution of actions as opposed to evaluation of outcomes, are defined early on as part of the blueprint that constructs this brain region, rather than sculpted through a later mechanism.
Graybiel and colleagues tracked what is happening early in development by driving cell-specific fluorescent markers that allowed them to follow the progenitors that give rise to cells in the striatum. The striatum is known, thanks to Graybiel’s early work, to be organized into compartments called striosomes and the matrix. These have distinct connections to other brain regions. Broadly speaking, while striosomes are linked to value-based decision-making and reinforcement-based behaviors, the matrix has been linked to action execution. These regions are further subdivided into direct and indirect pathways. The direct pathway neurons are involved in releasing inhibition in other regions of the basal ganglia and thus actively promote action. Neurons projecting into the indirect pathway, instead inhibit “unwanted” actions that are not part of the current “cortical plan.” Based on their tracking, Graybiel and colleagues were indeed able to build a “fate map” that told them when the cells that build these different regions of the striatum commit to a functional path during development.
“It was already well known that individual neurons have lineages that can be traced back to early development, and many such lineages are now being traced,” says Graybiel. “What is so striking in what we have found with the Huang lab is that the earliest specification of lineages we find—at least with the markers that we have used—corresponds to what later become the two major neurochemically distinct compartments of the striatum, rather than many other divisions that might have been specified first. If this is so, then the fundamental developmental ground plan of the striatum is expressed later by these two distinct compartments of the striatum.”
Building the striatum turns out to be a symphony of organization embedded in lateral ganglion eminence cells, the source of cells during development that will end up in the striatum. Progenitors made early in development are somewhat committed: they can only generate spiny projection neurons (SPNs) that are striosomal. Following this in time, cells that will give rise to matrix SPNs appear. There is then a second mechanism laid over this initial ground plan that is switched on in both striosomal and matrisomal neurons and independently gives rise to neurons that will connect into direct as opposed to indirect pathways. This latter specification of direct-indirect pathway neurons is less rigid, but there is an overarching tendency for neurons expressing a certain neurotransmitter, dopamine, to appear earlier in developmental time. In short, progenitors move through an orchestrated process where they generate spiny projection neurons that can first sit in any area of the striatum, then where the ultimate fate of cells is more restricted at the level of striosome or matrix, and finally choices are made in both regions regarding indirect-direct pathway circuitry. Remarkably, these results suggest that even at the very earliest development of the striatum, its ultimate organization is already laid down in a way that distinguishes value-related circuit from movement-related circuits.
“What is thrilling,” says Graybiel, “is that there are lineage progressions— the step by step laying out of the brain’s organization— the turn out to match the striosome-matrix architecture of the striatum the were not even known to exist 40 years ago!”
The striatum is a hub regulating movement, emotion, motivation, evaluation, and learning, and linked to disorders such as Parkinson’s Disease and persistent negative valuations. This means that understanding its construction has important implications, perhaps even, one day, for rebuilding a striatum affected by neurodegeneration. That said, the findings have broader implications. Consider the worm, specifically, C. elegans. The complete lineage of cells that make up this organism is known, including where each neuron comes from, what it connects to, and its function and phenotype. There’s a clear relationship between lineage and function in this relatively simple organism with its highly stereotyped nervous system. Graybiel’s work suggests that in the big picture, early development in the forebrain is also providing a game plan. In this case, however, this groundwork underpins for circuits that underlie extremely complex behaviors, those that come to support the volitional and habitual behaviors that make up part of who we are as individuals.