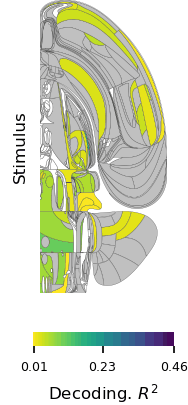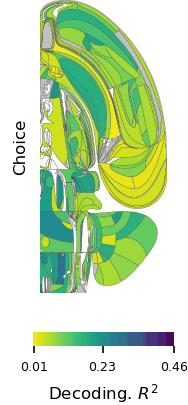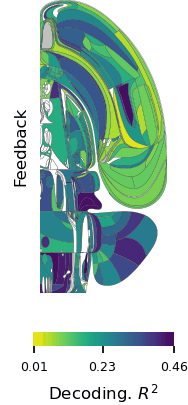
In a world full of competing sounds, we often have to filter out a lot of noise to hear what’s most important. This critical skill may come more easily for people with musical training, according to scientists at MIT’s McGovern Institute who used brain imaging to follow what happens when people try to focus their attention on certain sounds.
When Cassia Low Manting, a postdoctoral researcher working in the labs of McGovern Institute Investigators John Gabrieli and Dimitrios Pantazis, asked people to focus on a particular melody while another melody played at the same time, individuals with musical backgrounds were, unsurprisingly, better able to follow the target tune. An analysis of study participants’ brain activity suggests this advantage arises because musical training sharpens neural mechanisms that amplify the sounds they want to listen to while turning down distractions. “This points to the idea that we can train this selective attention ability,” Manting says.
The research team, including senior author Daniel Lundqvist at the Karolinska Institute in Sweden, reported their findings September 17, 2025, in the journal Science Advances. Manting, who is now at the Karolinska Institute, notes that the research is part of an ongoing collaboration between the two institutions.
Overcoming challenges
Participants in the study had vastly difference backgrounds when it came to music. Some were professional musicians with deep training and experience, while others struggled to differentiate between the two tunes they were played, despite each one’s distinct pitch. This disparity allowed the researchers to explore how the brain’s capacity for attention might change with experience. “Musicians are very fun to study because their brains have been morphed in ways based on their training,” Manting says. “It’s a nice model to study these training effects.”
Still, the researchers had significant challenges to overcome. It has been hard to study how the brain manages auditory attention, because when researchers use neuroimaging to monitor brain activity, they see the brain’s response to all sounds: those that the listener cares most about, as well as those the listener is trying to ignore. It is usually difficult to figure out which brain signals were triggered by which sounds.
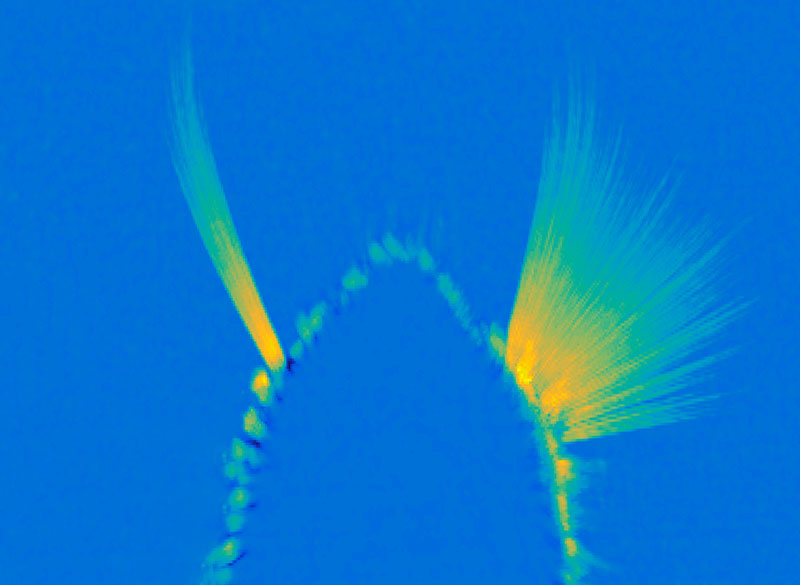
Manting and her colleagues overcame this challenge with a method called frequency tagging. Rather than playing the melodies in their experiments at a constant volume, the volume of each melody oscillated, rising and falling with a particular frequency. Each melody had its own frequency, creating detectable patterns in the brain signals that responded to it. “When you play these two sounds simultaneously to the subject and you record the brain signal, you can say, this 39-Hertz activity corresponds to the lower pitch sound and the 43-Hertz activity corresponds specifically to the higher pitch sound,” Manting explains. “It is very clean and very clear.”
When they paired frequency tagging with magnetoencephalography, a noninvasive method of monitoring brain activity, the team was able to track how their study participants’ brains responded to each of two melodies during their experiments. While the two tunes played, subjects were instructed to follow either the higher pitched or the lower pitched melody. When the music stopped, they were asked about the final notes of the target tune: did they rise or did they fall? The researchers could make this task harder by making the two tunes closer together in pitch, as well as by altering the timing of the notes.
Manting used a survey that asked about musical experience to score each participant’s musicality, and this measure had an obvious effect on task performance: The more musical a person was, the more successful they were at following the tune they had been asked to track.
To look for differences in brain activity that might explain this, the research team developed a new machine-learning approach to analyze their data. They used it to tease apart what was happening in the brain as participants focused on the target tune—even, in some cases, when the notes of the distracting tune played at the exact same time.
Top-down vs bottom-up attention
What they found was a clear separation of brain activity associated with two kinds of attention, known as top-down and bottom-up attention. Manting explains that top-down attention is goal-oriented, involving a conscious focus—the kind of attention listeners called on as they followed the target tune. Bottom-up attention, on the other hand, is triggered by the nature of the sound itself. A fire alarm would be expected to trigger this kind of attention, both with its volume and its suddenness. The distracting tune in the team’s experiments triggered activity associated with bottom-up attention—but more so in some people than in others.
“The more musical someone is, the better they are at focusing their top-down selective attention, and the less the effect of bottom-up attention is,” Manting explains.
Manting expects that musicians use their heightened capacity for top-down attention in other situations, as well. For example, they might be better than others at following a conversation in a room filled with background chatter. “I would put my bet on it that there is a high chance that they will be great at zooming into sounds,” she says.
She wonders, however, if one kind of distraction might actually be harder for a musician to filter out: the sound of their own instrument. Manting herself plays both the piano and the Chinese harp, and she says hearing those instruments is “like someone calling my name.” It’s one of many questions about how musical training affects cognition that she plans to explore in her future work.