From genes to brains
How advances in genomics will transform the study of brain function and disease.
Many brain disorders are strongly influenced by genetics, and researchers have long hoped that the identification of genetic risk factors will provide clues to the causes and possible treatments of these mysterious conditions. In the early years, progress was slow. Many claims failed to replicate, and it became clear that in order to identify the important risk genes with confidence, researchers would need to examine the genomes of very large numbers of patients.
Until recently that would have been prohibitively expensive, but genome research has been accelerating fast. Just how fast was underlined by an announcement in January from a California-based company, Illumina, that it had achieved a long-awaited milestone: sequencing an entire human genome for under $1000. Seven years ago, this task would have cost $10M and taken weeks of work. The new system does the job in a few hours, and can sequence tens of thousands of genomes per year.
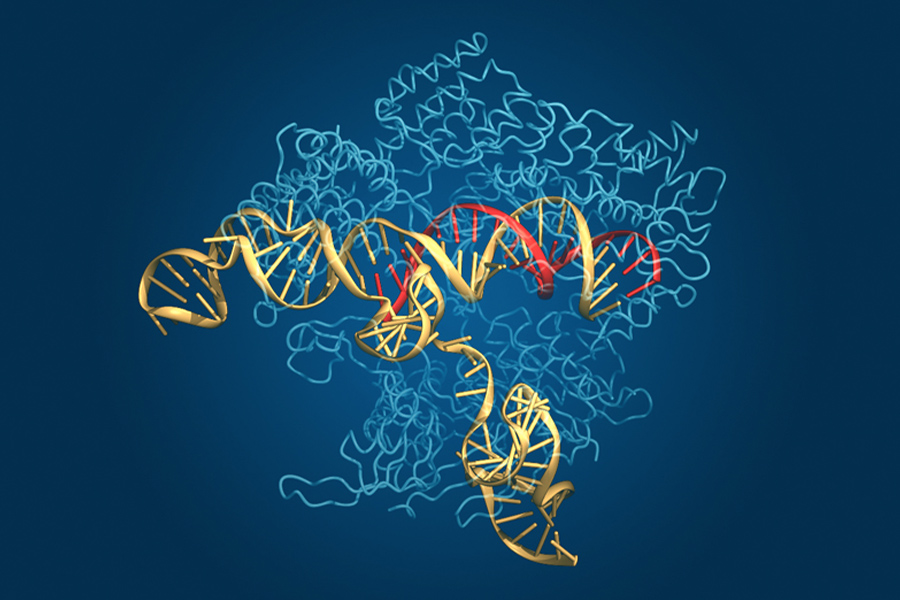
In parallel with these spectacular advances, another technological revolution has been unfolding over the past several years, with the development of a new method for editing the genome of living cells. This method, known as CRISPR, allows researchers to make precise changes to a DNA sequence—an advance that is expected to transform many areas of biomedical research and may ultimately form the basis of new treatments for human genetic disease.
The CRISPR technology, which is based on a natural bacterial defense system against viruses, uses a short strand of RNA as a “search string” to locate a corresponding DNA target sequence. This RNA string can be synthesized in the lab and can be designed to recognize any desired sequence of DNA. The RNA carries with it a protein called Cas9, which cuts the target DNA at the chosen location, allowing a new sequence to be inserted—providing researchers with a fast and flexible “search-and-replace” tool for editing the genome.
One of the pioneers in this field is McGovern Investigator Feng Zhang, who along with George Church of Harvard, was the first to show that CRISPR could be used to edit the human genome in living cells. Zhang is using the technology to study human brain disorders, building on the flood of new genetic discoveries that are emerging from advances in DNA sequencing. The Broad Institute, where Zhang holds a joint appointment, is a world leader in human psychiatric genetics, and will be among the first to acquire the new Illumina sequencing machines when they reach the market later this year.
By sequencing many thousands of individuals, geneticists are identifying the rare genetic variants that contribute to risk of diseases such as autism, schizophrenia and bipolar disorder. CRISPR will allow neuroscientists to study those gene variants in cells and in animal models. The goal, says McGovern Institute director Bob Desimone, is to understand the biological roots of brain disorders. “The biggest obstacle to new treatments has been our ignorance of fundamental mechanisms. But with these new technologies, we have a real opportunity to understand what’s wrong at the level of cells and circuits, and to identify the pressure points at which therapeutic intervention may be possible.”
Culture Club
In other fields, the influence of genetic variations on disease has turned out to be surprisingly difficult to unravel, and for neuropsychiatric disease, the challenge may be even greater. The brain is the most complex organ of the body, and the underlying pathologies that lead to disease are not yet well understood. Moreover, any given disorder may show a wide variation in symptoms from patient to patient, and it may also have many different genetic causes. “There are hundreds of genes that can contribute to autism or schizophrenia,” says McGovern Investigator Guoping Feng, who is also Poitras Professor of Neuroscience.
To study these genes, Feng and collaborators at the Broad Institute’s Stanley Center for Psychiatric Research are planning to screen thousands of cultures of neurons, grown in the tiny wells of cell culture plates. The neurons, which are grown from stem cells, can be engineered using CRISPR to contain the genetic variants that are linked to neuropsychiatric disease. Each culture will contain neurons with a different variant, and these will be examined for abnormalities that might be associated with disease.
Feng and colleagues hope this high-throughput platform will allow them to identify cellular traits, or phenotypes, that may be related to disease and which can then be studied in animal models to see if they cause defects in brain function or in behavior. In the longer term, this high-throughput platform can also be used to screen for new drugs that can reverse these defects.
Animal Kingdom
Cell cultures are necessary for large-scale screens, but ultimately the results must be translated into the context of brain circuits and behavior. “That means we must study animal models too,” says Feng.
Feng has created several mouse models of human brain disease by mutating genes that are linked to these disorders and examining the behavioral and cellular defects in the mutant animals. “We have models of obsessive-compulsive disorder and autism,” he explains. “By studying these mice we want to learn what’s wrong with their brains.”
So far, Feng has focused on single-gene models, but the majority of human psychiatric disorders are triggered by multiple genes acting in combination. One advantage of the new CRISPR method is that it allows researchers to introduce several mutations in parallel, and Zhang’s lab is now working to create autistic mice with more than one gene alteration.
Perhaps the most important advantage of CRISPR is that it can be applied to any species. Currently, almost all genetic modeling of human disease is restricted to mice. But while mouse models are convenient, they are limited, especially for diseases that affect higher brain functions and for which there are no clear parallels in rodents. “We also need to study species that are closer to humans,” says Feng.
Accordingly, he and Zhang are collaborating with colleagues in Oregon and China to use CRISPR to create primate models of neuropsychiatric disorders. Earlier this year, a team in China announced that they had used CRISPR to create transgenic monkeys that will be used to study defects in metabolism and immunity.
Feng and Zhang are planning to use a similar approach to study brain disorders, but in addition to macacques, they will also work with a smaller primate species, the marmoset. These animals, with their fast breeding cycles and complex behavioral repertoires, are ideal for genetic studies of behavior and brain function. And because they are very social with highly structured communication patterns, they represent a promising new model for understanding the neural basis of social cognition and its disruption in conditions such as autism.
Given their close evolutionary relationship to humans, marmoset models could also help accelerate the development of new therapies. Many experimental drugs for brain disorders have been tested successfully in mice, only to prove ineffective in subsequent human trials. These failures, which can be enormously expensive, have led many drug companies to cut back on their neuroscience R&D programs. Better animal models could reverse this trend by allowing companies to predict more accurately which drug candidates are most promising, before investing heavily in human clinical trials.
Feng’s mouse research provides an example of how this approach can work. He previously developed a mouse model of obsessive-compulsive disorder, in which the animals engage in obsessive self-grooming, and he has now shown that this effect can be reversed when the missing gene is reintroduced, even in adulthood. Other researchers have seemed similar results with other brain disorders such as Rett Syndrome, a condition that is often accompanied by autism. “The brain is amazingly plastic,” says Feng. “At least in a mouse, we have shown that the damage can often be repaired. If we can also show this in marmosets or other primate models, that would really give us hope that something similar is possible in humans.”
Human Race
Ultimately, to understand the genetic roots of human behavior, researchers must sequence the genomes of individual subjects in parallel with measurements of those same individuals’ behavior and brain function.
Such studies typically require very large sample sizes, but the plummeting cost of sequencing is now making this feasible. In China, for instance, a project is already underway to sequence the genomes of many thousands of individuals to uncover genetic influences on cognition and intelligence.
The next step will be to link the genetics to brain activity, says McGovern Investigator John Gabrieli, who also directs the Martinos Imaging Center at MIT. “It’s a big step to go from DNA to behavioral variation or clinical diagnosis. But we know those genes must affect brain function, so neuroimaging may help us to bridge that gap.”
But brain scans can be time-consuming, given that volunteers must perform behavioral tasks in the scanner. Studies are typically limited to a few dozen subjects, not enough to detect the often subtle effects of genomic variation.
One way to enlarge these studies, says Gabrieli, is to image the brain during rest rather than in a state of prompted activity. This procedure is fast and easy to replicate from lab to lab, and patterns of resting state activity have turned out to be surprisingly reproducible; moreover, Gabrieli is finding that differences in resting activity are associated with brain disorders such as autism, and he hopes that in the future it will be possible to relate these differences to the genetic factors that are emerging from genome studies at the Broad Institute and elsewhere.
“I’m optimistic that we’re going to see dramatic advances in our understanding of neuropsychiatric disease over the next few years.” — Bob Desimone
Confirming these associations will require a “big data” approach, in which results from multiple labs are consolidated into large repositories and analyzed for significant associations. Resting state imaging lends itself to this approach, says Gabrieli. “To find the links between brain function and genetics, big data is the direction we need to go to be successful.”
How soon might this happen? “It won’t happen overnight,” cautions Desimone. “There are a lot of dots that need to be connected. But we’ve seen in the case of genome research how fast things can move once the right technologies are in place. I’m optimistic that we’re going to see equally dramatic advances in our understanding of neuropsychiatric disease over the next few years.”