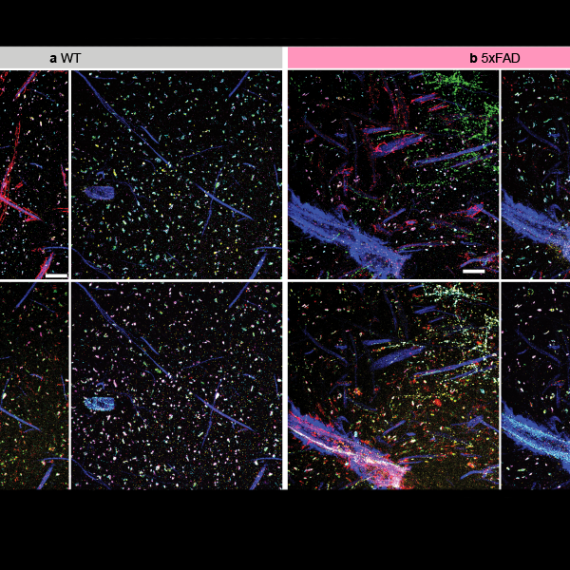
Explaining repetitive behavior linked to amphetamine use
Habit-forming drugs disrupt modulation of neurons linked to flexible behavior.
Repetitive movements such as nail-biting and pacing are very often seen in humans and animals under the influence of habit-forming drugs. Studies at the McGovern Institute have found that these repetitive behaviors may be due to a breakdown in communication between neurons in the striatum – a deep brain region linked to habit and movement, among other functions.
The Graybiel lab has a long-standing interest in habit formation and the effects of addiction on brain circuits related to the striatum, a key part of the basal ganglia. The Graybiel lab previously found remarkably strong correlations between gene expression levels in specific parts of the striatum and exposure to psychomotor stimulants such as amphetamine and cocaine. The longer the exposure to stimulant, the more repetitive behavior in models, and the more brain circuits changed. These findings held across animal models.
The lab has found that if they train animals to develop habits, they can completely block these repetitive behaviors using targeted inhibition or excitation of the circuits. They even could block repetitive movement patterns in a mouse model of obsessive-compulsive disorder (OCD). These experiments mimicked situations in humans in which drugs or anxiety-inducing experiences can lead to habits and repetitive movement patterns—from nail-biting to much more dangerous habitual actions.

Why would these circuits exist in the brain if they so often produce “bad” habits and destructive behaviors, as seen in compulsive use of drugs such as opioids or even marijuana? One answer is that we have to be flexible and ready to switch our behavior if something dangerous occurs in the environment. Habits and addictions are, in a way, the extreme pushing of this flexible system in the other direction, toward the rigid and repetitive.
“One important clue is that for many of these habits and repetitive and addictive behaviors, the person isn’t even aware that they are doing the same thing again and again. And if they are not aware, they can’t control themselves and stop,” explains Ann Graybiel, an Institute Professor at MIT. “It is as though the ‘rational brain’ has great difficulty in controlling the ‘habit circuits’ of the brain.” Understanding loss of communication is a central theme in much of the Graybiel lab’s work.
Graybiel, who is also a founding member of the McGovern Institute, is now trying to understand the underlying circuits at the cellular level. The lab is examining the individual components of the striatal circuits linked to selecting actions and motivating movement, circuits that seem to be directly controlled by drugs of abuse.
In groundbreaking early work, Graybiel discovered that the striatum has distinct compartments, striosomes and matrix. These regions are spatially and functionally distinct and separately connect, through striatal projection neurons (SPNs), to motor-control centers or to neurons that release dopamine, a neurotransmitter linked to all drugs of abuse. It is in these components that Graybiel and colleagues have more recently found strong effects of drugs. Indeed opposite changes in gene expression in the striosome SPNs versus the matrix SPNs, raises the possibility that an imbalance in gene regulation leads to abnormally inflexible behaviors caused by drug use.
“It was known that cholinergic interneurons tend to reside along the borders of the two striatal compartments, but whether this cell type mediates communication between the compartments was unknown,” explains first author Jill Crittenden, a research scientist in the Graybiel lab. “We wanted to know whether cholinergic signaling to the two compartments is disrupted by drugs that induce abnormally repetitive behaviors.”
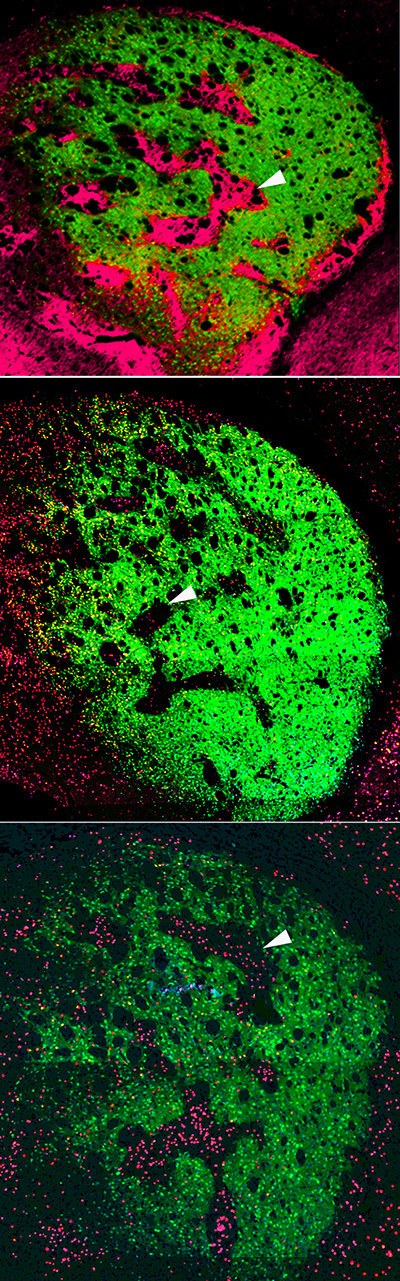
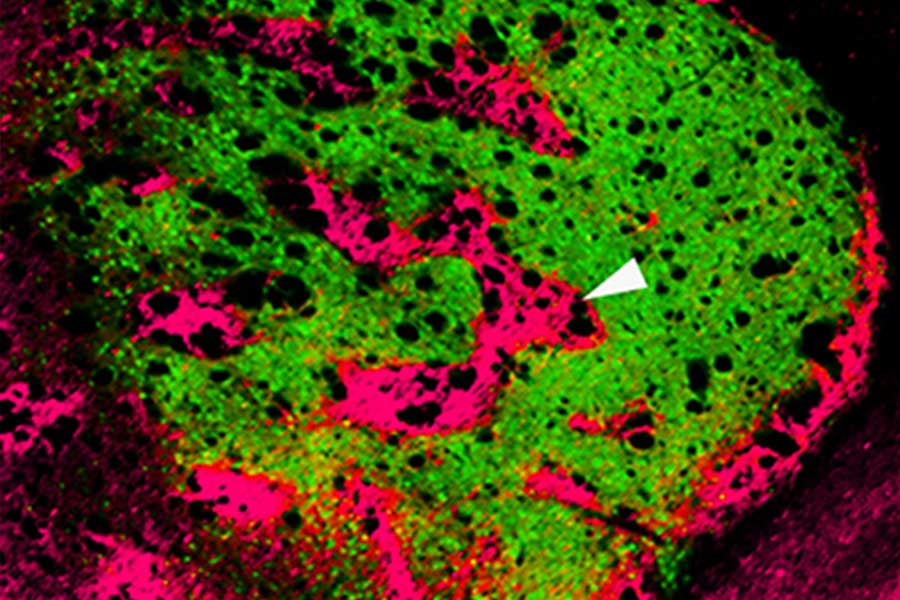
It was known that cholinergic interneurons are activated by important environmental cues and promote flexible rather than repetitive behavior, how this is related to interaction with SPNs in the striatum was unclear. “Using high-resolution microscopy,” explains Crittenden, “we could see for the first time that cholinergic interneurons send many connections to both striosome and matrix SPNs, well-placed to coordinate signaling directly across the two striatal compartments that appear otherwise isolated.”
Using a technique known as optogenetics, the Graybiel group stimulated mouse cholinergic interneurons and monitored the effects on striatal SPNs in brain tissue. They found that stimulating the interneurons inhibited the ongoing signaling activity that was induced by current injection in matrix and striatal SPNs. However, when examining the brains of animals on high doses of amphetamine and that were displaying repetitive behavior, stimulating the relevant interneurons failed to interrupt evoked activity in SPNs.
Using an inhibitor, the authors were able to show that these neural pathways depend on the nicotinic acetylcholine receptor. Inhibiting this cell-surface signaling receptor had a similar effect to drug intoxication on intercommunication among striatal neurons. Since break down of cholinergic interneuron signaling across striosome and matrix compartments under drug intoxication may reduce behavioral flexibility and cue responsiveness, the work suggests one mechanism for how drugs of abuse hijack action-selection systems of the brain and drive pathological habit-formation.
The Graybiel lab is excited that they can now manipulate these behaviors by manipulating very particular circuits components in the habit circuits. Most recently they have discovered that they can even fully block the effects of stress by manipulating cellular components of these circuits. They now hope to dive deep into these circuits to find out the mystery of how to control them.
“We hope that by pinpointing these circuit elements—which seem to have overlapping effects on habit formation, addiction and stress, we help to guide the development of better therapies for addiction,” explains Graybiel. “We hope to learn about what the use of drugs does to brain circuits with both short term use and long term use. This is an urgent need.”