Silent synapses are abundant in the adult brain
These immature connections may explain how the adult brain is able to form new memories and absorb new information.
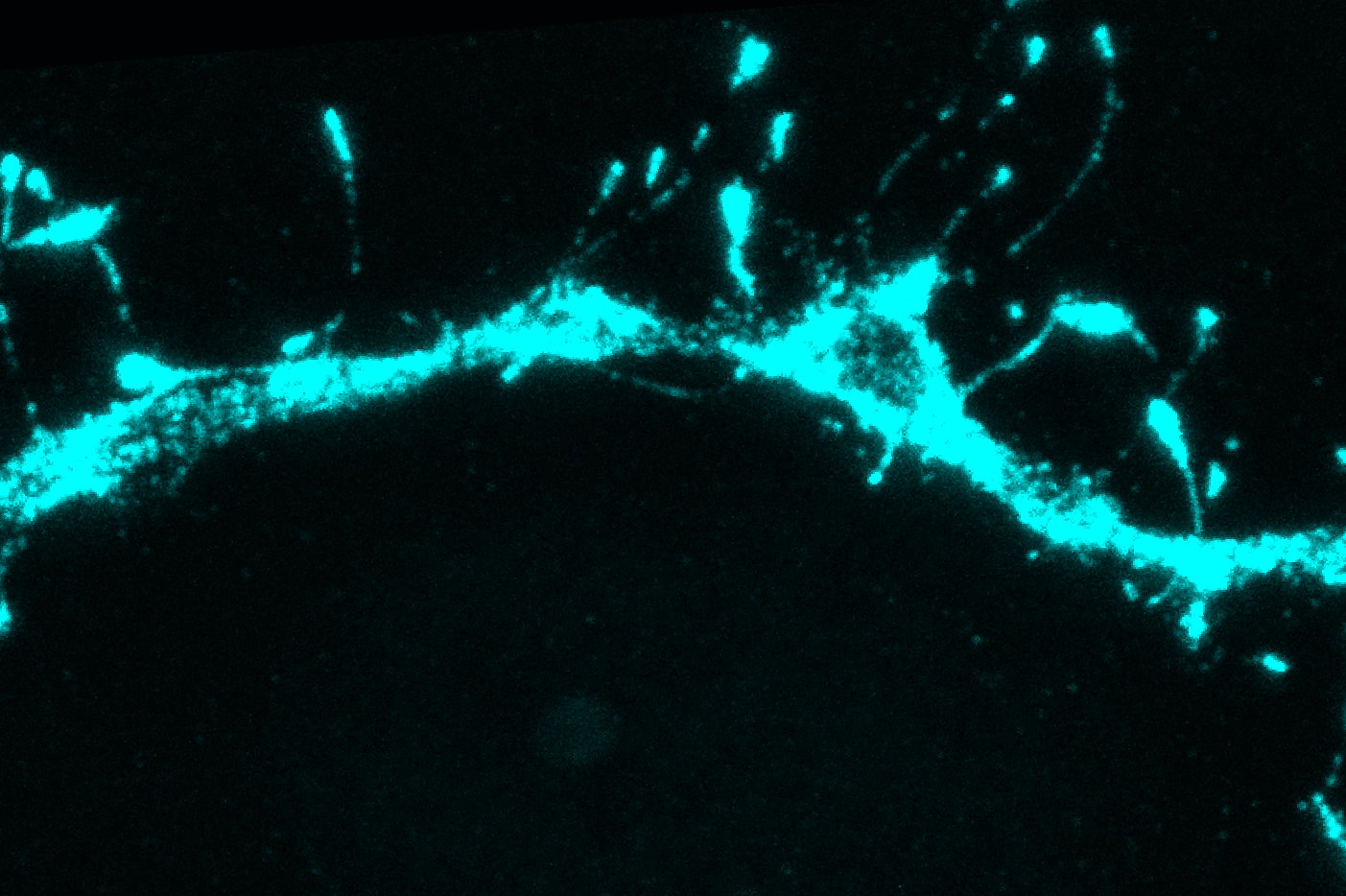
Until now, it was believed that silent synapses were present only during early development, when they help the brain learn the new information that it’s exposed to early in life. However, the new MIT study revealed that in adult mice, about 30 percent of all synapses in the brain’s cortex are silent.
The existence of these silent synapses may help to explain how the adult brain is able to continually form new memories and learn new things without having to modify existing conventional synapses, the researchers say.
“These silent synapses are looking for new connections, and when important new information is presented, connections between the relevant neurons are strengthened. This lets the brain create new memories without overwriting the important memories stored in mature synapses, which are harder to change,” says Dimitra Vardalaki, an MIT graduate student and the lead author of the new study.
Mark Harnett, an associate professor of brain and cognitive sciences and an investigator at the McGovern Institute for Brain Research, is the senior author of the paper, which appears today in Nature. Kwanghun Chung, an associate professor of chemical engineering at MIT, is also an author.
A surprising discovery
When scientists first discovered silent synapses decades ago, they were seen primarily in the brains of young mice and other animals. During early development, these synapses are believed to help the brain acquire the massive amounts of information that babies need to learn about their environment and how to interact with it. In mice, these synapses were believed to disappear by about 12 days of age (equivalent to the first months of human life).
However, some neuroscientists have proposed that silent synapses may persist into adulthood and help with the formation of new memories. Evidence for this has been seen in animal models of addiction, which is thought to be largely a disorder of aberrant learning.
Theoretical work in the field from Stefano Fusi and Larry Abbott of Columbia University has also proposed that neurons must display a wide range of different plasticity mechanisms to explain how brains can both efficiently learn new things and retain them in long-term memory. In this scenario, some synapses must be established or modified easily, to form the new memories, while others must remain much more stable, to preserve long-term memories.
In the new study, the MIT team did not set out specifically to look for silent synapses. Instead, they were following up on an intriguing finding from a previous study in Harnett’s lab. In that paper, the researchers showed that within a single neuron, dendrites — antenna-like extensions that protrude from neurons — can process synaptic input in different ways, depending on their location.
As part of that study, the researchers tried to measure neurotransmitter receptors in different dendritic branches, to see if that would help to account for the differences in their behavior. To do that, they used a technique called eMAP (epitope-preserving Magnified Analysis of the Proteome), developed by Chung. Using this technique, researchers can physically expand a tissue sample and then label specific proteins in the sample, making it possible to obtain super-high-resolution images.
The first thing we saw, which was super bizarre and we didn’t expect, was that there were filopodia everywhere.
While they were doing that imaging, they made a surprising discovery. “The first thing we saw, which was super bizarre and we didn’t expect, was that there were filopodia everywhere,” Harnett says.
Filopodia, thin membrane protrusions that extend from dendrites, have been seen before, but neuroscientists didn’t know exactly what they do. That’s partly because filopodia are so tiny that they are difficult to see using traditional imaging techniques.
After making this observation, the MIT team set out to try to find filopodia in other parts of the adult brain, using the eMAP technique. To their surprise, they found filopodia in the mouse visual cortex and other parts of the brain, at a level 10 times higher than previously seen. They also found that filopodia had neurotransmitter receptors called NMDA receptors, but no AMPA receptors.
A typical active synapse has both of these types of receptors, which bind the neurotransmitter glutamate. NMDA receptors normally require cooperation with AMPA receptors to pass signals because NMDA receptors are blocked by magnesium ions at the normal resting potential of neurons. Thus, when AMPA receptors are not present, synapses that have only NMDA receptors cannot pass along an electric current and are referred to as “silent.”
Unsilencing synapses
To investigate whether these filopodia might be silent synapses, the researchers used a modified version of an experimental technique known as patch clamping. This allowed them to monitor the electrical activity generated at individual filopodia as they tried to stimulate them by mimicking the release of the neurotransmitter glutamate from a neighboring neuron.
Using this technique, the researchers found that glutamate would not generate any electrical signal in the filopodium receiving the input, unless the NMDA receptors were experimentally unblocked. This offers strong support for the theory the filopodia represent silent synapses within the brain, the researchers say.
The researchers also showed that they could “unsilence” these synapses by combining glutamate release with an electrical current coming from the body of the neuron. This combined stimulation leads to accumulation of AMPA receptors in the silent synapse, allowing it to form a strong connection with the nearby axon that is releasing glutamate.
The researchers found that converting silent synapses into active synapses was much easier than altering mature synapses.
“If you start with an already functional synapse, that plasticity protocol doesn’t work,” Harnett says. “The synapses in the adult brain have a much higher threshold, presumably because you want those memories to be pretty resilient. You don’t want them constantly being overwritten. Filopodia, on the other hand, can be captured to form new memories.”
“Flexible and robust”
The findings offer support for the theory proposed by Abbott and Fusi that the adult brain includes highly plastic synapses that can be recruited to form new memories, the researchers say.
“This paper is, as far as I know, the first real evidence that this is how it actually works in a mammalian brain,” Harnett says. “Filopodia allow a memory system to be both flexible and robust. You need flexibility to acquire new information, but you also need stability to retain the important information.”
The researchers are now looking for evidence of these silent synapses in human brain tissue. They also hope to study whether the number or function of these synapses is affected by factors such as aging or neurodegenerative disease.
“It’s entirely possible that by changing the amount of flexibility you’ve got in a memory system, it could become much harder to change your behaviors and habits or incorporate new information,” Harnett says. “You could also imagine finding some of the molecular players that are involved in filopodia and trying to manipulate some of those things to try to restore flexible memory as we age.”
The research was funded by the Boehringer Ingelheim Fonds, the National Institutes of Health, the James W. and Patricia T. Poitras Fund at MIT, a Klingenstein-Simons Fellowship, and Vallee Foundation Scholarship, and a McKnight Scholarship.
Paper: "Filopodia are a structural substrate for silent synapses in adult neocortex"