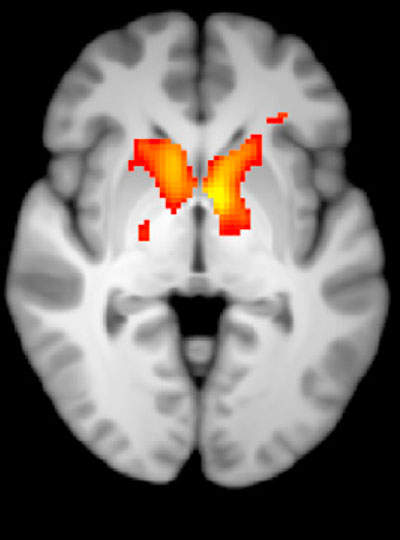
The lateral prefrontal cortex is a particularly well-connected part of the brain. Neurons there communicate with processing centers throughout the rest of the brain, gathering information and sending commands to implement executive control over behavior. Now, scientists at MIT’s McGovern Institute have mapped these connections and revealed an unexpected order within them: The lateral prefrontal cortex, they’ve found, contains maps of other major parts of the brain’s cortex.
The researchers, led by postdoctoral researcher Rui Xu and McGovern Institute Director Robert Desimone, report that the lateral prefrontal cortex contains a set of maps that represent the major processing centers in the other parts of the cortex, including the temporal and parietal lobes. Their organization likely supports the lateral prefrontal cortex’s roles managing complex functions such as attention and working memory, which require integrating information from multiple sources and coordinating activity elsewhere in the brain. The findings are published November 4, 2021, in the journal Neuron.
Topographic maps
The layout of the maps, which allows certain regions of the lateral prefrontal cortex to directly interact with multiple areas across the brain, indicates that this part of the brain is particularly well positioned for its role. “This function of integrating and then sending back control signals to appropriate levels in the processing hierarchies of the brain is clearly one of the reasons that prefrontal cortex is so important for cognition and executive control,” says Desimone.
In many parts of the brain, neurons’ physical organization has been found to reflect the information represented there. For example, individual neurons’ positions within the visual cortex mirror the layout of the cells in the retina from which they receive input, such that the spatial pattern of neuronal activity in this part of the brain provides an approximate view of the image seen by the eyes. For example, if you fixate on the first letter of a word, the next letters in the word will map to sequential locations in the visual cortex. Likewise, the arm and hand are mapped to adjacent locations in the somatic cortex, where the brain receives sensory information from the skin.
Topographic maps such as these, which have been found primarily in brain regions involved in sensory and motor processing, offer clues about how information is stored and processed in the brain. Neuroscientists have hoped that topographic maps within the lateral prefrontal cortex will provide insight into the complex cognitive processes that are carried out there—but such maps have been elusive.
Previous anatomical studies had given little indication how different parts of the brain communicate preferentially to specific locations within the prefrontal cortex to give rise to regional specialization of cognitive functions. Recently, however, the Desimone lab identified two areas within the lateral prefrontal cortex of monkeys with specific roles in focusing an animal’s visual attention. Knowing that some spots within the lateral prefrontal cortex were wired for specific functions, they wondered if others were, too. They decided they needed a detailed map of the connections emanating from this part of the brain, and devised a plan to plot connectivity from hundreds of points within the lateral prefrontal cortex.
Cortical connectome
To generate a wiring diagram, or connectome, Xu used functional MRI to monitor activity throughout a monkey’s brain as he stimulated specific points within its lateral prefrontal cortex. He moved systematically through the brain region, stimulating points spaced as close as one millimeter apart, and noting which parts of the brain lit up in response. Ultimately, the team collected data from about 100 sites for each of two monkeys.
As the data accumulated, clear patterns emerged. Different regions within the lateral prefrontal cortex formed orderly connections with each of five processing centers throughout the brain. Points within each of these maps connected to sites with the same relative positions in the distant processing centers. Because some parts of the lateral prefrontal cortex are wired to interact with more than one processing centers, these maps overlap, positioning the prefrontal cortex to integrate information from different sources.
The team found significant overlap, for example, between the maps of the temporal cortex, a part of the brain that uses visual information to recognize objects, and the parietal cortex, which computes the spatial relationships between objects. “It is mapping objects and space together in a way that would integrate the two systems,” explains Desimone. “And then on top of that, it has other maps of other brain systems that are partially overlapping with that—so they’re all sort of coming together.”
Desimone and Xu say the new connectome will help guide further investigations of how the prefrontal cortex orchestrates complex cognitive processes. “I think this really gives us a direction for the future, because we now need to understand the cognitive concepts that are mapped there,” Desimone says.
Already, they say, the connectome offers encouragement that a deeper understanding of complex cognition is within reach. “This topographic connectivity gives the lateral prefrontal some specific advantage to serve its function,” says Xu. “This suggests that lateral prefrontal cortex has a fine organization, just like the more studied parts of the brain, so the approaches that have been used to study these other regions may also benefit the studies of high-level cognition.”





 View the interactive version of this story in our
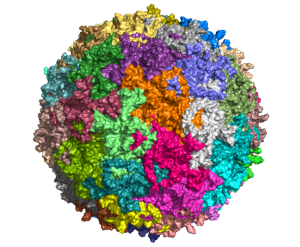
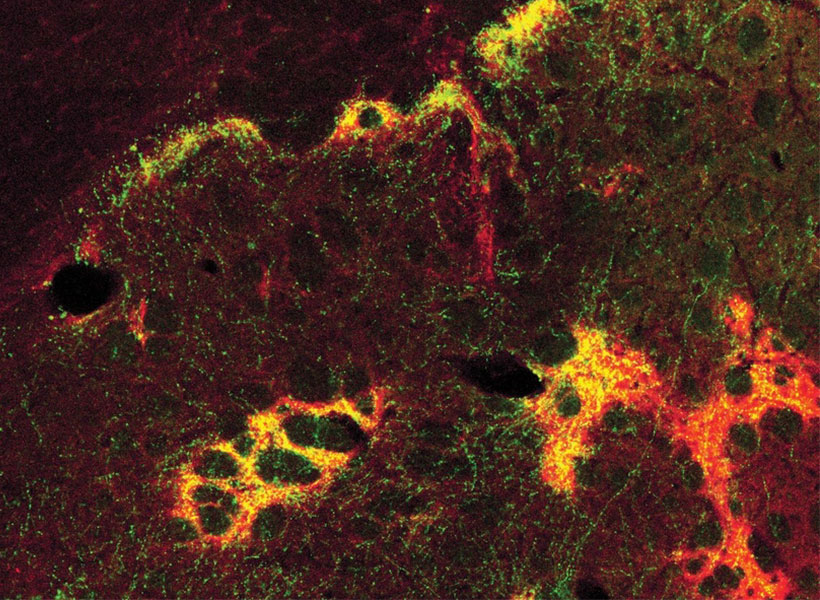
View the interactive version of this story in our  Taking advantage of its pernicious spread, neuroscientists use a modified version of the rabies virus to introduce a fluorescent protein to infected cells and visualize their connections (above). As a graduate student in Edward Callaway’s lab at the Salk Institute for Biological Studies, Wickersham figured out how to limit the virus’s passage through the nervous system, allowing it to access cells that are directly connected to the neuron it initially infects, but go no further. Rabies virus travels across synapses in the opposite direction of neuronal signals, so researchers can deliver it to a single cell or set of cells, then see exactly where those cells’ inputs are coming from.
Taking advantage of its pernicious spread, neuroscientists use a modified version of the rabies virus to introduce a fluorescent protein to infected cells and visualize their connections (above). As a graduate student in Edward Callaway’s lab at the Salk Institute for Biological Studies, Wickersham figured out how to limit the virus’s passage through the nervous system, allowing it to access cells that are directly connected to the neuron it initially infects, but go no further. Rabies virus travels across synapses in the opposite direction of neuronal signals, so researchers can deliver it to a single cell or set of cells, then see exactly where those cells’ inputs are coming from. In Feng’s lab, research scientist Martin Wienisch is working to make it easier to control this aspect of delivery. Rather than relying on the genetic makeup of an entire animal to determine where a virally-transported gene is switched on, instructions can be programmed directly into the virus, borrowing regulatory sequences that cells already know how to interpret.
In Feng’s lab, research scientist Martin Wienisch is working to make it easier to control this aspect of delivery. Rather than relying on the genetic makeup of an entire animal to determine where a virally-transported gene is switched on, instructions can be programmed directly into the virus, borrowing regulatory sequences that cells already know how to interpret. Enhancers will also be useful for delivering potential gene therapies to patients, Wienisch says. For many years, the Feng lab has been studying how a missing copy of a gene called Shank3 impairs neurons’ ability to communicate, leading to autism and intellectual disability. Now, they are investigating whether they can overcome these deficits by delivering a functional copy of Shank3 to the brain cells that need it. Widespread activation of the therapeutic gene might do more harm than good, but incorporating the right enhancer could ensure it is delivered to the appropriate cells at the right dose, Wienisch says.
Enhancers will also be useful for delivering potential gene therapies to patients, Wienisch says. For many years, the Feng lab has been studying how a missing copy of a gene called Shank3 impairs neurons’ ability to communicate, leading to autism and intellectual disability. Now, they are investigating whether they can overcome these deficits by delivering a functional copy of Shank3 to the brain cells that need it. Widespread activation of the therapeutic gene might do more harm than good, but incorporating the right enhancer could ensure it is delivered to the appropriate cells at the right dose, Wienisch says.