Nature: An unexpected source of innovative tools to study the brain
McGovern scientists look beyond neuroscience to find materials and inspiration for innovative tools and techniques to study the brain.
This story originally appeared in the Fall 2023 issue of BrainScan.
___
In their quest to deepen their understanding of the brain, McGovern scientists take inspiration wherever it comes — and sometimes it comes from surprising sources. To develop new tools for research and innovative strategies for treating disease, they’ve drawn on proteins that organisms have been making for billions of years as well as sophisticated materials engineered for modern technology.
For McGovern investigator Feng Zhang, the natural world provides a rich source of molecules with remarkable and potentially useful functions.
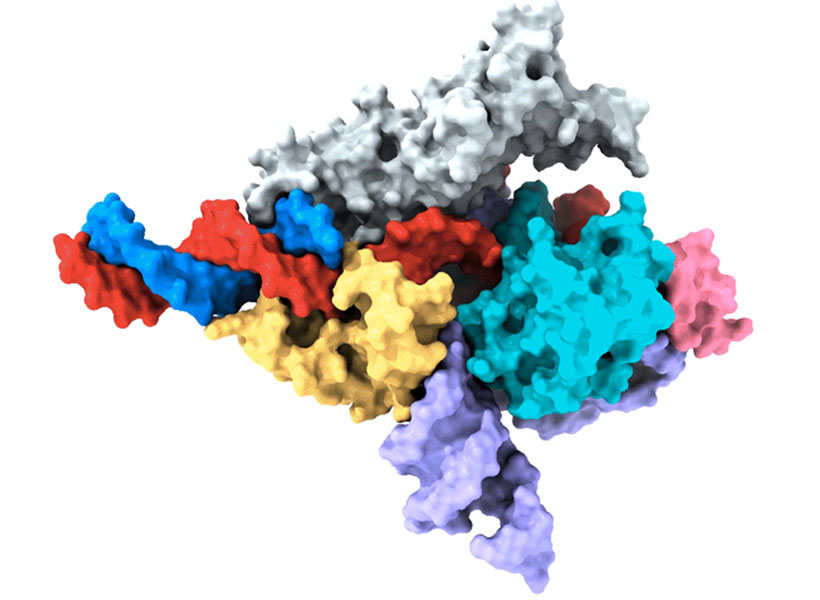
Zhang is one of the pioneers of CRISPR, a programmable system for gene editing that is built from the components of a bacterial adaptive immune system. Scientists worldwide use CRISPR to modify genetic sequences in their labs, and many CRISPR-based therapies, which aim to treat disease through gene editing, are now in development. Meanwhile, Zhang and his team have continued to explore CRISPR-like systems beyond the bacteria in which they were originally discovered.
Turning to nature
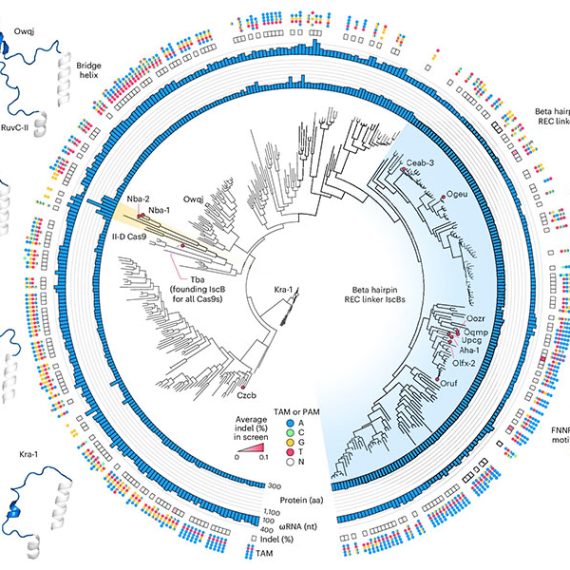
This year, the search for evolutionarily related systems led Zhang’s team to a set of enzymes made by more complex organisms, including single-celled algae and hard-shell clams. Like the enzymes that power CRISPR, these newly discovered enzymes, called Fanzors, can be directed to cut DNA at specific sites by programming an RNA molecule as a guide.
Rhiannon Macrae, a scientific advisor in Zhang’s lab, says the discovery was surprising because Fanzors don’t seem to play the same role in immunity that CRISPR systems do. In fact, she says it’s not clear what Fanzors do at all. But as programmable gene editors, Fanzors might have an important advantage over current CRISPR tools — particularly for clinical applications. “Fanzor proteins are much smaller than the workhorse CRISPR tool, Cas9,” Macrae says. “This really matters when you actually want to be able to use one of these tools in a patient, because the bigger the tool, the harder it is to package and deliver to patients’ cells.”
Zhang’s team has thought a lot about how to get therapies to patients’ cells, and size is only one consideration. They’ve also been looking for ways to direct drugs, gene-editing tools, or other therapies to specific cells and tissues in the body. One of the lab’s leading strategies comes from another unexpected natural source: a microscopic syringe produced by certain insect-infecting bacteria.
In their search for an efficient system for targeted drug delivery, Zhang and graduate student Joseph Kreitz first considered the injection systems of bacteria-infecting viruses: needle-like structures that pierce the outer membrane of their host to deliver their own genetic material. But these viral injection systems can’t easily be freed from the rest of the virus.
Then Zhang learned that some bacteria have injection systems of their own, which they release inside their hosts after packing them with toxins. They reengineered the bacterial syringe, devising a delivery system that works on human cells. Their current system can be programmed to inject proteins — including those used for gene editing — directly into specified cell types. With further development, Zhang hopes it will work with other types of therapies, as well.
Magnetic imaging
In McGovern Associate Investigator Alan Jasanoff’s lab, researchers are designing sensors that can track the activity of specific neurons or molecules in the brain, using magnetic resonance imaging (MRI) or related forms of non-invasive imaging. These tools are essential for understanding how the brain’s cells and circuits work together to process information. “We want to give MRI a suite of metaphorical colors: sensitivities that enable us to dissect the different kinds of mechanistically significant contributors to neural activity,” he explains.
Jasanoff can tick off a list of molecules with notable roles in biology and industry that his lab has repurposed to glean more information from brain imaging. These include manganese — a metal once used to tint ancient glass; nitric oxide synthase — the enzyme that causes blushing; and iron oxide nanoparticles — tiny magnets that enable compact data storage inside computers. But Jasanoff says none of these should be considered out of place in the imaging world. “Most are pretty logical choices,” he says. “They all do different things and we use them in pretty different ways, but they are either magnetic or interact with magnetic molecules to serve our purposes for brain imaging.”

The enzyme nitric oxide synthase, for example, plays an important role in most functional MRI scans. The enzyme produces nitric oxide, which causes blood vessels to expand. This can bring a blush to the cheeks, but in the brain, it increases blood flow to bring more oxygen to busy neurons. MRI can detect this change because it is sensitive to the magnetic properties of blood.
By using blood flow as a proxy for neural activity, functional MRI scans light up active regions of the brain, but they can’t pinpoint the activity of specific cells. So Jasanoff and his team devised a more informative MRI sensor by reengineering nitric oxide synthase. Their modified enzyme, which they call NOSTIC, can be introduced into a select group of cells, where it will produce nitric oxide in response to neural activity — triggering increased blood flow and strengthening the local MRI signal. Researchers can deliver it to specific kinds of brain cells, or they can deliver it exclusively to neurons that communicate directly with one another. Then they can watch for an elevated MRI signal when those cells fire. This lets them see how information flows through the brain and tie specific cells to particular tasks.
Miranda Dawson, a graduate student in Jasanoff’s lab, is using NOSTIC to study the brain circuits that fuel addiction. She’s interested in the involvement of a brain region called the insula, which may mediate the physical sensations that people with addiction experience during drug cravings or withdrawal. With NOSTIC, Dawson can follow how the insula communicates to other parts of the brain as a rat experiences these MITstages of addiction. “We give our sensor to the insula, and then it projects to anatomically connected brain regions,” she explains. “So we’re able to delineate what circuits are being activated at different points in the addiction cycle.”

Mining biodiversity
McGovern investigators know that good ideas and useful tools can come from anywhere. Sometimes, the key to harnessing those tools is simply recognizing their potential. But there are also opportunities for a more deliberate approach to finding them.
McGovern Investigator Ed Boyden is leading a program that aims to accelerate the discovery of valuable natural products. Called the Biodiversity Network (BioNet), the project is collecting biospecimens from around the world and systematically analyzing them, looking for molecular tools that could be applied to major challenges in science and medicine, from brain research to organ preservation. “The idea behind BioNet,” Boyden explains, “is rather than wait for chance to give us these discoveries, can we go look for them on purpose?”