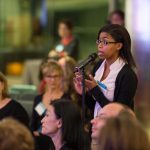On October 14, 2011, Nobel laureates David Hubel and Torsten Wiesel discussed their early explorations of the visual cortex.
Category: Uncategorized
Brain Scan Cover Image: Summer 2013
White matter fiber tracts in the adult human brain. Image: Zeynep Saygin
Brain Scan Cover Image: Spring 2013
A bundle of spiny apical dendrites, reconstructed from a series of ultra-thin slices of mouse cortex. Image: Daniel Berger and Sebastian Seung (MIT); based on data from Jeff Lichtman and colleagues (Harvard).
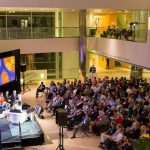
Brains on Trial with Alan Alda
What if we could peer into the brain to determine guilt or innocence? Could advances in neuroscience help reform our criminal justice system?
On Tuesday, September 17th, the McGovern Institute hosted a discussion with a distinguished group of legal and neuroscience experts who debated these and related questions. Alan Alda moderated the panel of experts, showed clips from his 2-part PBS special, “Brains on Trial,” and engaged the audience in a Q&A session.
See below for a photo gallery of the event. All photos courtesy of Justin Knight.
Brains on Trial with Alan Alda
What if we could peer into the brain to determine guilt or innocence? Could advances in neuroscience help reform our criminal justice system?
On Tuesday, September 17th, the McGovern Institute hosted a discussion with a distinguished group of legal and neuroscience experts who debated these and related questions. Alan Alda moderated the panel of experts, showed clips from his 2-part PBS special, “Brains on Trial,” and engaged the audience in a Q&A session.
Feng Zhang named to Popular Science Brilliant 10
Popular Science magazine has named two MIT junior faculty members — Pedro Reis and Feng Zhang — to its 2013 Brilliant 10 list of young stars in science and technology. The list will appear in the magazine’s October issue.
“Popular Science prides itself on revealing the innovations and ideas that are laying today’s groundwork for tomorrow’s breakthroughs, and the Brilliant 10 is one of the most exciting ways we do that,” says Jake Ward, editor-in-chief. “This collection of 10 brilliant young researchers is our chance to honor the most promising work — and the most hardworking people — in science and technology today. This year’s winners are particularly distinguished and I’m proud to welcome them all as members of the 2013 Brilliant 10.”
Pedro Reis, the Esther and Harold E. Edgerton Assistant Professor of Civil and Environmental Engineering and Mechanical Engineering, studies the mechanics of slender structures, with a particular focus on devising new ways of turning mechanical failure into functionality.
Over the past few years, Reis, 35, has published a number of eclectic and impactful papers in prominent journals. In 2009 he reported on the delamination of thin films adhered to soft foundations, which is relevant for stretchable electronics. He explained why adhesive films tear into triangular shapes, a problem that applies to both the everyday peeling of adhesive tape from a roll and the manufacturing of tapered graphene nanoribbons. Motivated by the closing of aquatic flowers, he recently discovered a new mechanism for passively pipetting liquids using a petal-shaped object. And last year inspired by a toy, Reis introduced the Buckliball, a new class of structures that uses buckling to provide origami-like folding capabilities to curved structures with potential uses for encapsulation and soft robotics.
In other work undertaken just for fun, Reis and colleagues reported in 2010 that when cats lap fluids (milk or water, for example), they take advantage of a perfect balance between gravity and inertia.
Feng Zhang, 31, is the W.M. Keck Career Development Professor in Biomedical Engineering, an assistant professor in the department of Brain and Cognitive Sciences, a member of the McGovern Institute for Brain Research and a core member of the Broad Institute. He received the award for his work on genome editing. Earlier this year he reported a powerful new way to make targeted mutations in genomic DNA, based on a bacterial system known as CRISPR. The new method will greatly accelerate the development of animal models of human genetic diseases, and may eventually make it possible to correct genetic mutations in patients. Zhang, a pioneer in optogenetics, has also recently invented a new method for controlling gene expression with light, in which light-sensitive plant proteins are engineered to create an “optical switch” that can turn other genes on or off at will.
This is the 12th annual Brilliant 10 list. Ten MIT researchers were included on previous lists.
Nanodiamonds
The Boyden lab is exploring the use of fluorescent nanodiamonds as a new class of optical probes for neuroscience research. Photo: Justin Knight
Dyslexia ‘seen in brain scans’ of preschool children
John Gabrieli’s lab has found that differences in a key language structure can be seen even before children start learning to read.
The study was picked up by various news outlets including the BBC, CBS, WBUR, US News and World Report, the UK Daily Mail, Fox News, and more.
Visualizing the Brain
Zeynep Saygin, a postdoc in Nancy Kanwisher’s lab uses a technology known as diffusion-weighted MR imaging to reveal long-range connections in the brain.
Tracking the roots of reading ability
Researchers in the Gabrieli lab have found that differences in a key language structure can be seen even before children start learning to read.