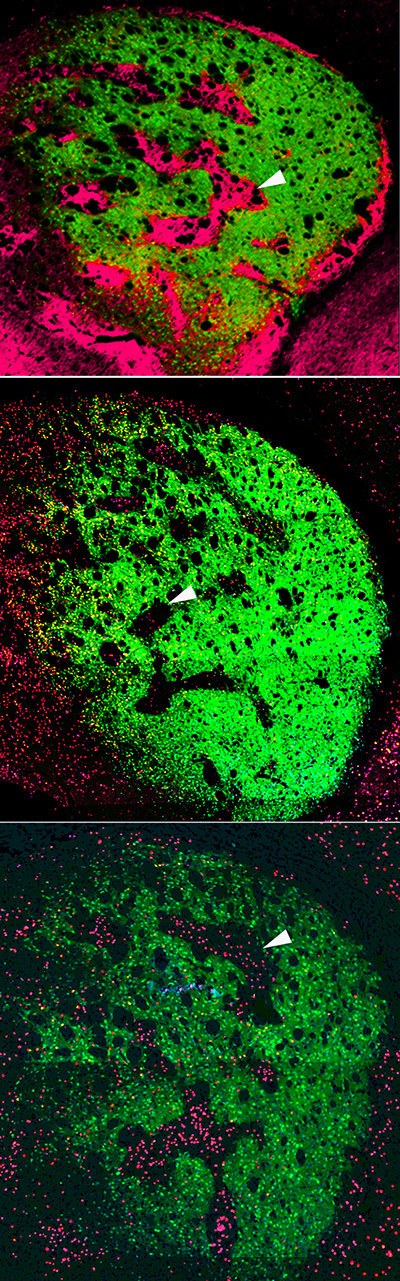
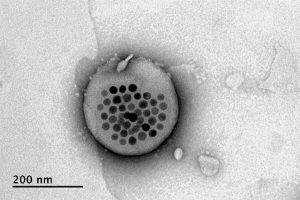
This story originally appeared in the Fall 2022 issue of BrainScan.
***
Many neuroscientists were drawn to their careers out of curiosity and wonder. Their deep desire to understand how the brain works drew them into the lab and keeps them coming back, digging deeper and exploring more each day. But for some, the work is more personal.
Several McGovern faculty say they entered their field because someone in their lives was dealing with a brain disorder that they wanted to better understand. They are committed to unraveling the basic biology of those conditions, knowing that knowledge is essential to guide the development of better treatments.
The distance from basic research to clinical progress is shortening, and many young neuroscientists hope not just to deepen scientific understanding of the brain, but to have direct impact on the lives of patients. Some want to know why people they love are suffering from neurological disorders or mental illness; others seek to understand the ways in which their own brains work differently than others. But above all, they want better treatments for people affected by such disorders.
Seeking answers
That’s true for Kian Caplan, a graduate student in MIT’s Department of Brain and Cognitive Sciences who was diagnosed with Tourette syndrome around age 13. At the time, learning that the repetitive, uncontrollable movements and vocal tics he had been making for most of his life were caused by a neurological disorder was something of a relief. But it didn’t take long for Caplan to realize his diagnosis came with few answers.

Tourette syndrome has been estimated to occur in about six of every 1,000 children, but its neurobiology remains poorly understood.
“The doctors couldn’t really explain why I can’t control the movements and sounds I make,” he says. “They couldn’t really explain why my symptoms wax and wane, or why the tics I have aren’t always the same.”
That lack of understanding is not just frustrating for curious kids like Caplan. It means that researchers have been unable to develop treatments that target the root cause of Tourette syndrome. Drugs that dampen signaling in parts of the brain that control movement can help suppress tics, but not without significant side effects. Caplan has tried those drugs. For him, he says, “they’re not worth the suppression.”
Advised by Fan Wang and McGovern Associate Director Guoping Feng, Caplan is looking for answers. A mouse model of obsessive-compulsive disorder developed in Feng’s lab was recently found to exhibit repetitive movements similar to those of people with Tourette syndrome, and Caplan is working to characterize those tic-like movements. He will use the mouse model to examine the brain circuits underlying the two conditions, which often co-occur in people. Broadly, researchers think Tourette syndrome arises due to dysregulation of cortico-striatal-thalamo-cortical circuits, which connect distant parts of the brain to control movement. Caplan and Wang suspect that the brainstem — a structure found where the brain connects to the spinal cord, known for organizing motor movement into different modules — is probably involved, too.
Wang’s research group studies the brainstem’s role in movement, but she says that like most researchers, she hadn’t considered its role in Tourette syndrome until Caplan joined her lab. That’s one reason Caplan, who has long been a mentor and advocate for students with neurodevelopmental disorders, thinks neuroscience needs more neurodiversity.
“I think we need more representation in basic science research by the people who actually live with those conditions,” he says. Their experiences can lead to insights that may be inaccessible to others, he says, but significant barriers in academia often prevent this kind of representation. Caplan wants to see institutions make systemic changes to ensure that neurodiverse and otherwise minority individuals are able to thrive in academia. “I’m not an exception,” he says, “there should be more people like me here, but the present system makes that incredibly difficult.”
Overcoming adversity
Like Caplan, Lace Riggs faced significant challenges in her pursuit to study the brain. She grew up in Southern California’s Inland Empire, where issues of social disparity, chronic stress, drug addiction, and mental illness were a part of everyday life.

“Living in severe poverty and relying on government assistance without access to adequate education and resources led everyone I know and love to suffer tremendously, myself included,” says Riggs, a postdoctoral fellow in the Feng lab.
“There are not a lot of people like me who make it to this stage,” says Riggs, who has lost friends and family members to addiction, mental illness, and suicide. “There’s a reason for that,” she adds. “It’s really, really difficult to get through the educational system and to overcome socioeconomic barriers.”
Today, Riggs is investigating the origins of neurodevelopmental conditions, hoping to pave the way to better treatments for brain disorders by uncovering the molecular changes that alter the structure and function of neural circuits.
Riggs says that the adversities she faced early in life offered valuable insights in the pursuit of these goals. She first became interested in the brain because she wanted to understand how our experiences have a lasting impact on who we are — including in ways that leave people vulnerable to psychiatric problems.
“While the need for more effective treatments led me to become interested in psychiatry, my fascination with the brain’s unique ability to adapt is what led me to neuroscience,” says Riggs.
After finishing high school, Riggs attended California State University in San Bernardino and became the only member of her family to attend university or attempt a four-year degree. Today, she spends her days working with mice that carry mutations linked to autism or ADHD in humans, studying the animals’ behavior and monitoring their neural activity. She expects that aberrant neural circuit activity in these conditions may also contribute to mood disorders, whose origins are harder to tease apart because they often arise when genetic and environmental factors intersect. Ultimately, Riggs says, she wants to understand how our genes dictate whether an experience will alter neural signaling and impact mental health in a long-lasting way.
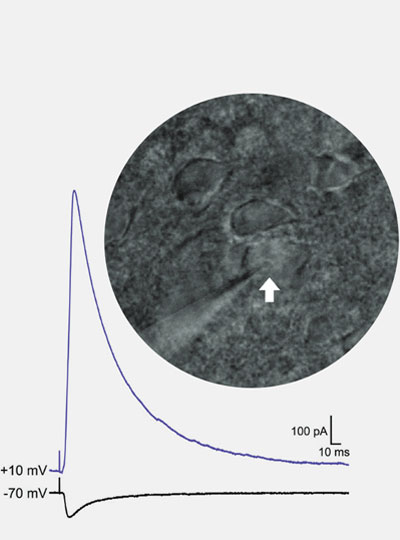
“If we understand how these long-lasting synaptic changes come about, then we might be able to leverage these mechanisms to develop new and more effective treatments.”
While the turmoil of her childhood is in the past, Riggs says it is not forgotten — in part, because of its lasting effects on her own mental health. She talks openly about her ongoing struggle with social anxiety and complex post-traumatic stress disorder because she is passionate about dismantling the stigma surrounding these conditions. “It’s something I have to deal with every day,” Riggs says. That means coping with symptoms like difficulty concentrating, hypervigilance, and heightened sensitivity to stress. “It’s like a constant hum in the background of my life, it never stops,” she says.
“I urge all of us to strive, not only to make scientific discoveries to move the field forward,” says Riggs, “but to improve the accessibility of this career to those whose lived experiences are required to truly accomplish that goal.”